hcooch ch2 h2o Unlocking the Secrets of a Simple Yet Powerful Chemical Reaction
Have you ever wondered how everyday chemicals can transform through a straightforward process to create useful products? Well, that’s exactly what happens with hcooch ch2 h2o. This intriguing notation represents the hydrolysis of methyl formate, a reaction where methyl formate breaks down in the presence of water to produce formic acid and methanol. It’s a classic example of how nature’s building blocks can be rearranged in optimistic ways to benefit industries and science. In this article, we’ll dive deep into hcooch ch2 h2o, exploring its components, mechanisms, and real-world impacts. By the end, you’ll see why this reaction holds such promise for a brighter, more efficient future in chemistry.
Understanding the Basics of hcooch ch2 h2o
At its core, hcooch ch2 h2o is all about change and adaptation. Picture this: a small molecule like methyl formate meets water, and together they create something new. The formula hcooch ch2 h2o is a shorthand way chemists describe this interaction, where “” stands for the ester group in methyl formate (properly written as HCOOCH3), “” hints at the carbon connections involved, and “” is simply water. Don’t let the notation scare you off—it’s like a puzzle that, once solved, reveals elegant simplicity.
This reaction falls under ester hydrolysis, a process that’s been studied for years because it’s so reliable. In everyday terms, it’s like mixing ingredients in a kitchen to bake a cake; each part plays a role, and the outcome is predictable yet exciting. What makes optimistic is its reversibility—under the right conditions, you can go back and forth, making it versatile for lab work and factories alike.
- Why it matters: shows how basic elements can lead to advanced applications.
- Historical note: Discovered in the early days of organic chemistry, it paved the way for modern synthesis methods.
To visualize the structure of methyl formate central to :
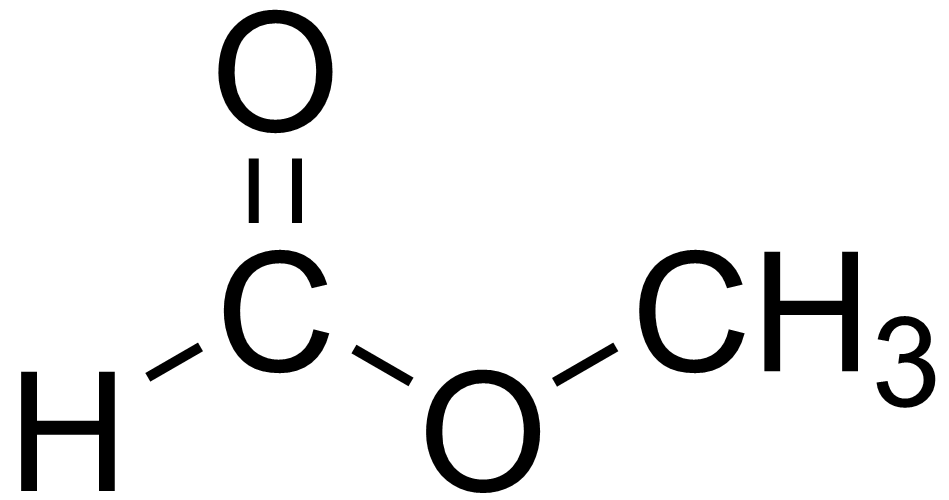
The Key Components in hcooch ch2 h2o
Breaking it down, hcooch ch2 h2o involves three main players: methyl formate, a methylene-like group in the structure, and water. Methyl formate, or HCOOCH3, is a colorless liquid with a sweet smell, much like fruit. It’s the starting point, and when it reacts with water, magic happens.
Water, of course, is the unsung hero here. Without it, wouldn’t occur. It’s abundant, cheap, and eco-friendly, which is why this reaction gets thumbs up from green chemists. The “ch2” part refers to the carbon-hydrogen bonds that shift during the process, acting like a bridge between atoms.
Think of it as a team effort—each component brings something to the table, creating balance. This synergy is what makes a go-to for producing acids and alcohols efficiently.
How the Hydrolysis Reaction Works in hcooch ch2 h2o
Now, let’s get into the nitty-gritty. The hydrolysis in hcooch ch2 h2o can happen in two ways: acid-catalyzed or base-catalyzed. In the acid version, a bit of acid speeds things up, protonating the ester so water can attack more easily. It’s like giving a push to a swing to make it go higher.
The base-catalyzed path, on the other hand, uses a base to pull things apart gently. Either way, the end result is formic acid (HCOOH) and methanol (CH3OH). The equation looks like this: HCOOCH3 + H2O → HCOOH + CH3OH.
What’s optimistic about this? It’s clean and doesn’t produce harmful waste if done right. Plus, it can be scaled up for big industries without much fuss.
Here’s an illustration of a typical ester hydrolysis reaction like in :
Production Methods Related to hcooch ch2 h2o
Producing the starting materials for is straightforward. Methyl formate comes from methanol and carbon monoxide under pressure, with a base as a helper. It’s efficient, with high yields that make economists smile.
On the flip side, reversing formate from formic acid and methanol—is how labs often prepare it. This back-and-forth is like a dance, showing the reaction’s flexibility.
- Industrial scale: Factories use carbonylation for mass production.
- Lab tips: Add a catalyst to speed things up safely.
Properties of the Compounds in hcooch ch2 h2o
Each part of has unique traits. Methyl formate boils at a low 32°C, making it volatile but useful as a solvent. It’s flammable, so handle with care, but its low toxicity is a plus.
Formic acid, one product, is pungent and used in preservatives. Methanol is clear and mixes well with water, ideal for fuels.
| Compound | Boiling Point (°C) | Density (g/cm³) | Common Use |
| Methyl Formate | 32 | 0.98 | Solvent |
| Formic Acid | 101 | 1.22 | Preservative |
| Methanol | 65 | 0.79 | Fuel |
| Water | 100 | 1.00 | Solvent/Catalyst |
This table highlights why is practical—affordable and accessible properties all around.
Industrial Applications of hcooch ch2 h2o
hcooch ch2 h2o shines in industry. Formic acid from this reaction preserves animal feed, keeping it fresh longer. That’s huge for farming, reducing waste and boosting efficiency.
Methanol powers cars and makes plastics. Imagine a world where fuels are cleaner thanks to reactions like this—it’s happening now!
- In textiles: Formic acid helps dye fabrics evenly.
- In energy: Methanol fuels cells for portable power.
The optimistic side? These uses promote sustainability, cutting down on pollution.
Environmental Benefits of hcooch ch2 h2o
Speaking of green, is a winner. Methyl formate replaces harmful blowing agents in foams, with zero global warming potential. It’s like swapping out old light bulbs for LEDs—better for the planet.
Formic acid from aids in eco-friendly leather tanning, reducing chrome use. And methanol blends make gasoline less smoky.
This reaction encourages a hopeful shift toward biodegradable products and renewable resources.
Safety Considerations in Handling hcooch ch2 h2o
While exciting, requires caution. Methyl formate is flammable, so good ventilation is key. Methanol can be toxic if ingested, but proper gear prevents issues.
- Tips: Wear gloves and goggles; store in cool places.
- Emergency: Rinse eyes if exposed; seek medical help.
With smart practices, risks drop, letting us focus on the positives.
Future Prospects for hcooch ch2 h2o
Looking ahead, hcooch ch2 h2o could revolutionize biofuels and pharmaceuticals. Researchers are tweaking catalysts for faster, greener versions. It’s like upgrading a bike to an e-bike—same idea, more power.
In a world pushing for sustainability, this reaction’s low cost and efficiency make it a star. Who knows? It might power the next big innovation.
FAQs
What exactly does hcooch ch2 h2o represent?
It stands for the hydrolysis reaction where methyl formate (HCOOCH3) reacts with water (H2O) to form formic acid and methanol.
Is hcooch ch2 h2o safe for everyday use?
In controlled settings, yes, but always follow safety guidelines due to flammability and toxicity risks.
How is hcooch ch2 h2o used in farming?
Formic acid from the reaction preserves silage, helping farmers store feed effectively.
Can help the environment?
Absolutely—it leads to eco-friendly products like low-emission fuels and non-toxic preservatives.
Why study today?
It offers insights into sustainable chemistry, promising better industrial processes for tomorrow.
Conclusion
In wrapping up, hcooch ch2 h2o isn’t just a chemical notation—it’s a gateway to innovative, sustainable solutions. From its simple mechanism to wide-ranging applications, this reaction embodies optimism in science. Whether in labs or factories, continues to inspire, proving that even basic interactions can lead to extraordinary outcomes. As we move forward, embracing such processes will surely brighten our chemical future.




